Injection Machines
6Performance-driven injection molding machines.

Extrusion Machines
3Versatile plastic extrusion systems.
Auxiliary Equipment
6Streamlined automation solutions for every step in plastics manufacturing.

Parts & Service
5Parts, Service, Digital, and Rebuild & Retrofit.

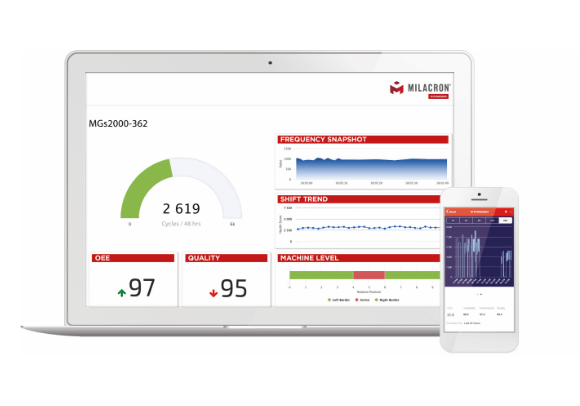
Digital Solutions
1IIOT Platforms that turn data into a competitive advantage.
Tech Support
Training and service designed to minimize downtime and improve efficiency.


